This paper summarizes the direct observation of tagged hydroxyl within 200 μs after its formation in the temperature range of 294 K–891 K. We estimate the time and temperature frame of the coexistence of chemical reactions and diffusion based on analysis of the behavior of the tagging fluorescence spot of photodissociation hydroxyl. The reasons for the existence of chemical reactions and interactions between chemical reactions and diffusion are then analyzed. Theoretical and experimental identification of this frame can be explored for non-contact spectral measurements based on hydroxyl in a general flow field.
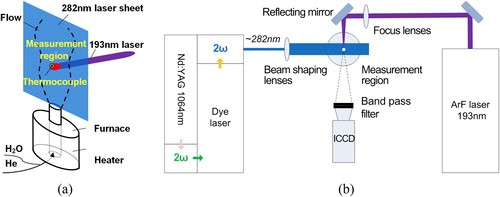
Schematic of the experimental system for observing the OH movement characteristics: the (a) measurement region and (b) experimental setup.
https://aip.scitation.org/doi/10.1063/5.0030979
Download: