The projective angiograph, jointly developed by XIOPM, CAS and its joint-stock company-Vivolight Medical Device & Technology Co., Ltd., made its first appearance on the International Annual Conference Upon Phlebology, Frankfurt 2014 and drew attentions of European medical experts.
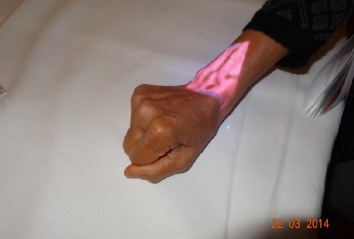
The projective angiograph is a medical testing device that applied to mainline. Since hemoglobin absorbs near-infrared light at a rate different from that of other tissues, projective angiograph casts blood vessels near the skin upon the skin through infrared backlight scattering, image capture, image process and micro-projection technology, etc., which can clearly show the subdermal vascular network. The device may assist medical workers in assessing superficial vessels safely, effectively and intuitively and finding the most suitable vessel for puncture conveniently and quickly, which will greatly improve the puncture efficiency and its success rate, decrease medical workers’ labor intensity, ease nurses’ mental stress and cut down medical disputes caused due to puncture treatment.
International Annual Conference Upon Phlebology, Frankfurt dates from 1987. In March each year medical experts who are mostly from Europe will gather in Frankfurt for giving lectures and exchanging ideas with experts from the world on this grand international conference. The professional exhibition upon industries of phlebology and lymphology will also be held over the same period.
On the academic annual conference, the projective angiograph was tested by medical experts from Germany, Ukraine, Mexico and other countries. Many vascular surgery physicians experienced the projector on site and highly praised to it. They left contact information and showed their intention for cooperation.
As Vivolight Medical Device & Technology Co., Ltd. has passed TUV Rheinland ISO13485 Medical Device Quality Management System Certification (TüV is the mark of safety certification granted by German TüV for component products and is widely approved in Europe). The projective angiograph will gradually expand the European market and XIOPM and Vivolight Medical Device & Technology Co., Ltd. will keep concentrating on scientific research and product innovation in the field of OCT optical image technology and make contributions to China’s high-end medical optical image technology and national health.


